Viennese Chikungunya Vaccine Now Approved from Age Twelve
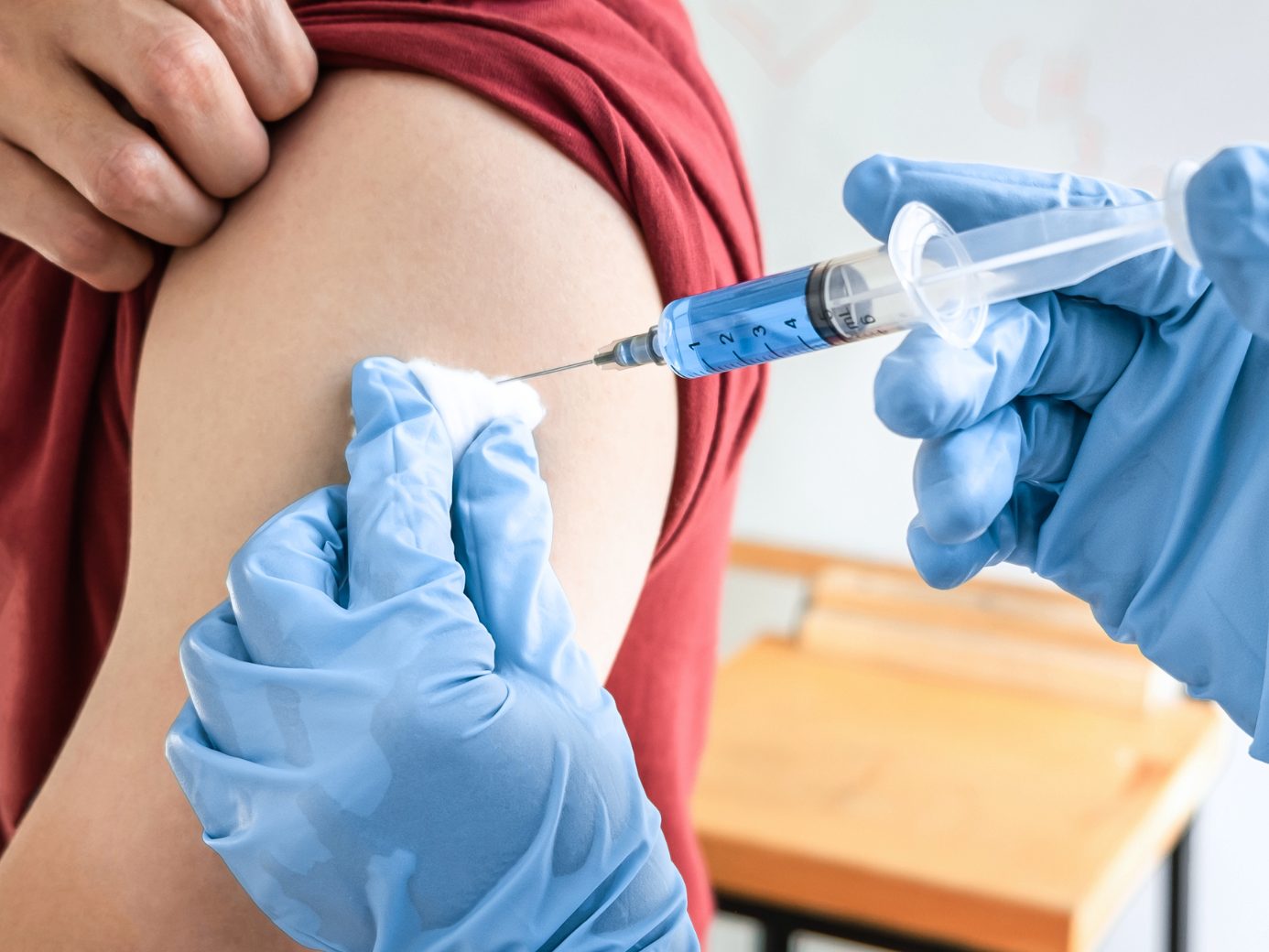
This extension complements the market authorization for adults, which was granted in July 2024, reported the French-Austrian pharmaceutical company on Tuesday evening. Applications for extending the authorization to adolescents have also been submitted in the USA, Canada, and the United Kingdom, according to the announcement.
Chikungunya virus causes joint pain, among other symptoms
The Chikungunya virus causes joint pain, among other symptoms, which is often debilitating and can last from weeks to years. In 2004, the disease began to spread rapidly. Since then, Chikungunya has been detected in more than 110 countries in Asia, Africa, Europe, and the Americas. Recently, for example, there was a larger outbreak on the French island of La Réunion off Madagascar. The vaccine is administered as a single dose and is recommended in Austria for travelers to endemic areas.
(APA/Red)
This article has been automatically translated, read the original article here.
Du hast einen Hinweis für uns? Oder einen Insider-Tipp, was bei dir in der Gegend gerade passiert? Dann melde dich bei uns, damit wir darüber berichten können.
Wir gehen allen Hinweisen nach, die wir erhalten. Und damit wir schon einen Vorgeschmack und einen guten Überblick bekommen, freuen wir uns über Fotos, Videos oder Texte. Einfach das Formular unten ausfüllen und schon landet dein Tipp bei uns in der Redaktion.
Alternativ kannst du uns direkt über WhatsApp kontaktieren: Zum WhatsApp Chat
Herzlichen Dank für deine Zusendung.


